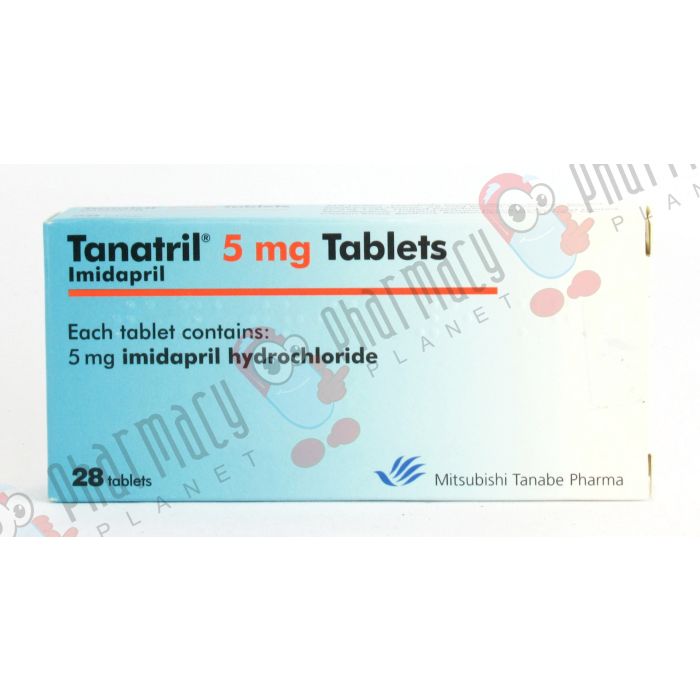
Tanatril
Tanatril is indicated in the treatment of high blood pressure (hypertension). Tanatril belongs to a group of medicines called ACE inhibitors (an enzyme that converts angiotensin). If you have high blood pressure, Tanatril will work by dilating the blood vessels, so that the blood circulates more easily. How blood pressure depends on the diameter of the blood vessels, its Blood pressure will drop with the help of Tanatril. It will also be easier for your heart to pump the blood through the vessels to the entire body.
the items you wish
to order

by our qualified
prescriber
to your door step
Please use the options provided to make your final selection.
| Brand | Medicine Strength | Size | Price |
|---|---|---|---|
| - | 5mg | 28 Tablets | 38.00 |
| - | 5mg | 56 Tablets | 50.00 |
| - | 5mg | 84 Tablets | 59.00 |
| - | 10mg | 28 Tablets | 38.00 |
| - | 10mg | 56 Tablets | 50.00 |
| - | 10mg | 84 Tablets | 59.00 |
| - | 20mg | 28 Tablets | 38.00 |
| - | 20mg | 56 Tablets | 50.00 |
| - | 20mg | 84 Tablets | 59.00 |
It looks like you missed filling in questions from the last consultation.Click Here to submit your response.

It is the prescribers duty to ensure that the medicine is being used safely and appropriately. please answer a few questions about yourself to help us process your request.
Customer Service Team
Confidentiality & Authenticity Assured
- Fully Regulated UK Pharmacy
- Authentic UK sourced medicines only.
- Trading for more than 10 years.
- Information Governance lead ensures all data is confidential
Need Help With Your Questionnaire?
Our customer service team is on hand Monday-Friday to help you with your queries.
Please call us on 08009788956 or
email us at: headoffice@pharmacyplanet.com
Tanatril is indicated in the treatment of high blood pressure (hypertension). Tanatril belongs to a group of medicines called ACE inhibitors (an enzyme that converts angiotensin).










